
Exhibition time: 17-19 March, 2025 Shanghai, China
 中文
中文

Exhibition time: 17-19 March, 2025 Shanghai, China
 中文
中文

Types of anti-caking agents
An anticaking agent is a material added to the fertiliser to promote the maintenance of its good physical condition during storage and handling. There are two main groups, namely coating agents and internal additives.
The internal additives are added during the production of the fertiliser and they act internally as hardeners or crystal modifiers resulting in improved storage properties. Examples are the addition of formaldehyde to molten urea, magnesium nitrate solution to ammonium nitrate, and the presence or addition of impurities, such as iron and aluminium in wet process phosphoric acid production.
Coating agents are applied to the surface of the fertiliser particles and can be classified according to several criteria depending on type and application, e.g. inert powders, liquid coating agents and solid coating agents.
These additives, which were the first anticaking agents in technical use, are applied as fine powders. Examples are dolomite, diatomaceous earth (kieselguhr), kaolin (basic aluminium silicate) and talc (basic magnesium silicate) which function as mechanical barriers between the particles. There are some distinct disadvantages in using inert powders alone. The main disadvantage is the need of very high dosages (2-3 % w/w) connected to the problem of low adherence, resulting in heavy dusting from the treated product. The protection against ambient humidity is also rather low. Nowadays such treatments are not used. A better adherence of powders can be reached by combining it in a two-step treatment with an oil or even better with a coating agent (Mackay and Sharples, 1985). Such combinations are normally used on complex fertilisers. The function of a powder could also be to reduce the stickiness of the fertiliser granules to avoid clogging of the conveyor belt.
These types of agents are based on organic surface-active agents and non-surface-active agents (Anon, 1993). For better spreading, the active components are in most cases dissolved in mineral oil but also in water or (poly) glycols.
Surfactant molecules comprise a non-polar hydrophobic (water-repellent) group and one or more polar hydrophilic (water-loving) groups. They are divided into three groups according to the charge carried by their hydrophilic portion: anionics, cationics and non-ionics. Anionic if the charge is negative, cationic if positive and non-ionic when the molecule is neutral.
The cationic surfactants are dominated by the fatty amines, especially those with a long carbon chain. The type most often used is a hydrogenated tallow alkyl amine with a main chain length of C16-C18. These cationics are the most common organic surfactants used in anticaking treatments and are considered to be one of the more effective additives known (Thompson, 1972; Neville, 1978).
They are oil-soluble surfactants that adhere to the granule surface and make it hydro-phobic, thus improving the water repellence. They also affect the crystal structure and suppress the formation of contact area as well as reduce the tensile strength of the bond between the granules.
Fatty amines with their strong adsorption characteristics have proved to be the most efficient crystal structure modifiers. Not only are they outstanding with ammonium nitrate and ammonium nitrate-based products, they are in fact the only surfactant with sufficient anticaking efficiency. Their performance is a result of the exchange of ammonia and the incorporation of the fatty ammonium ion into the crystal lattice. The amine can also be successfully combined with other crystal modifiers for treating products of greater complexity (Anon, 1999a).
The non-ionic surfactants are also dominated by a single class of compounds, the polyoxyethylene condensates. They might still be used as anticaking agents for complex fertilisers, but only in combination with other surfactants.
Another class is compounds that are not surface-active, but simply hydrophobic and examples of these non-surface-active coating agents are paraffin waxes, synthetic polymers and mineral oils. A barrier is created between the granules preventing wetting across the contact points and delaying the absorption of moisture from the ambient air.
This group of coating agents is applied in solid form, prills or flakes, for melt application on hot fertiliser. Examples are the use of amine acetate and hydrogenated fatty amine for treatment of potassium chloride (KCl).
To act as an anticaking agent, an additive has to interfere with the caking mechanism. There are a great number of possible ways that this can happen and the following reasons for the effect of various anticaking agents have been proposed in the literature (Rosenblom and Zettervall, 1978; Thompson, 1972; Hoffmeister, 1979):
o Prevention of wetting across the contact points of the granules.
o Diffusion of the crystallising phase over the granule surface.
o Nucleation of small fine crystals during dissolution/re-crystallisation processes.
o Modification of crystal structure and size of crystals formed during dissolution/re-crystallisation processes.
o Inhibition of dissolution/re-crystallisation processes.
o Spreading of the liquid film over the granule surface, promoted by reduction of surface tension, thus reducing the formation of droplets of liquid at the granule surface, as well as reducing condensation in the capillary formed at the contact points.
o Reduction of formation of contact areas.
o Reduction of tensile strength of the bond between the granules.
o Removal of moisture from the granule surface by absorption.
o Protection from ambient humidity.
Traditional paraffin-based coatings are usually composed of:
o Oil.
o Paraffins (or waxes).
o Alkylamine.
o Optionally other special additives.
o Possibly in combination with an inert powder (e.g. talcum, chalk, clay, gypsum).
Several kinds of oil are successfully applied in fertiliser coatings, ranging from white oils to fuel oil. Oils mainly consist of branched and cyclic alkanes as well as polycyclic aromatics. All these components are strongly hydrophobic. The oils differ, amongst other factors, in their viscosity and solidification point. Viscous oils can be applied to bind dust or inert powder coating.
Paraffins comprise a mixture of linear and branched alkanes. These compounds are hydrophobic. High linear alkane contents can be found in paraffin waxes. By selecting specific mixtures of paraffins and oil the optimum solidification and distribution properties can be tailor-made for every fertiliser production unit.
It has been observed that the composition of the paraffin mixture is crucial for either anticaking or water repellent properties: Anticaking agents require relatively high amounts of branched alkanes, whereas only linear alkanes give water repellent activity (Bijpost, 2003). In general good water repellent coatings possess moderate anticaking properties and vice versa.
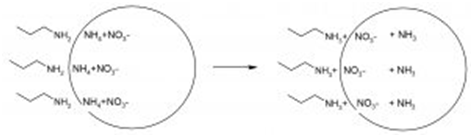
Most of the anticaking agents comprise a fatty amine, usually a tallow amine. There are several theories about the function of the alkylamine (Ohlsson, 2000). First of all it can act as a surface active agent; it can contribute to an optimal distribution of the paraffin/oil carrier over the granules. Secondly, an alkylamine can react with ammonium salts under exchange of ammonia (Figure 1). It leads to a strong adhesion of the coating to the granule.
The surface modification after moisture uptake has a significant effect on the caking performance. Freshly treated fertiliser with either fatty acid or fatty amine based coatings can show a similar protection against caking. After a moisture-cycle, the caking tendency of these samples appeared to be completely different: the amine-based coating retains more or less its original protection, whereas the performance of the coating with the fatty acid collapses.
Fatty amines have been mixed with all kind of ‘special additives’. Well-known is the addition of fatty acids to alkylamine/oil based coatings. The combination of alkylamine/carbonic acid is widely applied to adjust the rheology properties of liquids (Bieleman, 2000). For oils and paraffins it means that the mixture becomes more viscous.
Alkylcarboxylates, such as aluminium stearate, are generally used as anti caking agents (from candy to fertiliser). They can be admixed with alkylamines to improve the anticaking behaviour.
Mixtures of aromatic sulphonates and alkylamines can be applied as anticaking coating as well. The sulphonates as such can be applied as an aqueous solution. These products have been found to be especially suitable for the treatment of potassium nitrate. If using aqueous sulphonates for other fertiliser types, dust formation after moisture pick up can occur.
Alkylamine/alkylphosphate ester salts are added to linear alkanes to improve the moisture repellent properties of the coated fertiliser particles (Goethals, 1984). These products appeared to be excellent coating agents for hygroscopic (phase stabilised) ammonium nitrates. Long term bulk storage of treated ammonium nitrate leads only to a thin crust on the bulk. The underlying nitrate granules remain free flowing and intact.
Due to the health and environmental issues associated with paraffin-based coatings, new types of fertiliser coatings have been developed that meet the following criteria:
o Mineral oil (and possibly paraffin) free.
o Low toxic nature.
o Acceptable dosages.
o Biodegradability.
o Reasonable price.
o Competitive treatment costs.
Only a limited number of product groups have been identified which give the desired performance, these being ones based on vegetable oils (and derivatives), synthetic biodegradable polymers and natural by-products other than vegetable oils. Also developed were coatings to reduce the oil absorption of ammonium nitrates.
Due to the name ‘vegetable oils’, it is often thought that these are the ultimate alternative for mineral oils. However, vegetable oils (and several animal oils, such as fish oil, lard and tallow) mainly consist of glycerol triesters from C12-C24 fatty acids. These products are readily biodegradable. The ester function can be hydrolysed in both acidic and alkaline media under formation of fatty acid and glycerol. In addition, every vegetable oil contains various double (unsaturated) bonds. These moieties as such are sensitive to oxidation, cross-link and/or polymerisation reactions (e.g. Lawson, 1994).
After prolonged storage such reactions have led to rancidity of the oil and an annoying odour. The rate of this process is strongly increased at elevated temperatures. In addition, it has been found that iron can catalyse the cross-link and polymerisation reactions. These conditions can finally result in very viscous to insoluble products. There are plenty of examples of tube blocking and complete destruction of tanks caused by reactions of natural oils.
Mineral oils mainly consist of branched and cyclic alkanes as well as polycyclic aromates. These compounds do not possess polar groups at all. The difference in performance between mineral and vegetable oils becomes especially clear in moisture uptake tests: vegetable oil-based products do not show any water repellence on ammonium nitrates. Apparently, the presence of the polar groups makes the vegetable oil-coated products less hydrophobic or the moisture permeability of vegetable oils is higher than that of paraffin.
It has been found that mixtures of alkylcarboxylates and vegetable oils or alkylesters of fatty acids are capable of binding dust and of decreasing the caking propensity of several fertiliser grades (Spence, 2006). The alkyl esters useful are chains consisting of 6 to 24 carbon atoms containing 1 to 3 double bonds.
Coatings based on synthetic biodegradable polymers.
Caking of ammonium nitrate-containing fertilisers can be reduced by the application of polyalkylene glycols (Mekog-Albatros, 1966). Most polyalkylene glycols are soluble in water, show low toxicity and are biologically degradable. The optimum dosages are in the range of between 0.3 and 1.0 weight percent based on the weight of the treated fertiliser. These dosages are significantly higher than applied for oil/paraffin-containing products.
It has been claimed that biodegradable polyamines can be mixed with polyalkylene glycols to give good anticaking performance for ammonium nitrates, NPK and urea (Bijpost, 2006). Industrial tests have demonstrated that coatings based on biodegradable synthetic polymers can give a long term performance on calcium ammonium nitrate: even after six months the treated fertiliser is still free-flowing. The dust formation is comparable with that obtained with the conventional treatment.
When using biodegradable coatings there were certain doubts about microbiological growth with storage time. This might lead to the formation of, for example, fungus on the fertiliser surface. However, no microbiological activity on the surface of the granules could be observed. Apparently, the high salt concentration at the interface of the fertiliser and the biodegradable coating prevents microbiological growth.
For some fertiliser types, the treatment costs with the biopolymer-based coatings can be higher than with the traditional coatings. In addition, the performance against moisture uptake is less sufficient than that of a traditional water repellent coating. However, most coatings explored are liquid at room temperature and have a very low solidification point, making them convenient for handling.
Coatings based on natural by-products.
Vegetable by-products originate usually from potatoes, grain, soya and sugar beet. Renewable animal-based products are, for example, bones, lard and tallow. Owing to several purification steps, the latter raw materials are relatively expensive. Moreover, the odour can be annoying. Vegetable-based renewable products are very available and cheap. Molasses is frequently applied as a colouring agent for fertilisers. The variety in vegetable by-products is wide. The selection can take place on the basis of contents of:
o Dry material.
o Starch.
o Protein.
o Fat.
o Amino acids (and various types of amino acids).
As solids are difficult to use directly as coating, the dry material must be dissolved or dispersed in a carrier, preferably water. To diminish the negative effects of water, it is worthwhile to focus on highly concentrated vegetable products.
It has been claimed that protein-rich materials from renewable sources can provide good anticaking protection for several fertilisers, such as ammonium nitrates and urea (Bijpost, 2006). With addition of biodegradable polymers the performance can further be adjusted, if desired. Industrial pilot tests have shown that coatings based on natural by-products can give an anticaking performance comparable to the traditional coatings. As these new products are cheap and available, they form an attractive environmentally friendly alternative to the conventional anticaking agents. A drawback can be the very characteristic smell.
Even at low dosages these new coatings display a good performance. When applying these types of coatings it has been found that good anticaking behaviour can be coupled with fine water repellent properties. This special characteristic differs from the conventional paraffin/oil-based coatings as well. It can open the way to develop a full range of new fertiliser treatment products, meeting today’s highest environmental criteria.
![1691659713130506.png RN0$0UK(HNCT%_P7BI`9K]O.png](/sdhg/Public/ueditor/php/upload/image/20230810/1691659713130506.png)
Equally important as the choice of a coating agent, are the application method and the dosage rate of the coating agent to ensure a good performance.
Important factors to consider are the dosage system, equipment used for spraying the liquid coating agent, the temperature of the agent and of the fertiliser granules in the application moment, plus the performance of the coating drum.
Primarily a dosage system consists of a storage tank with heating facilities. It is important that heating coils are well placed to ensure that the contents of the tank are equally heated. If not, care must be taken to install a stirrer or to pump around the product in the storage tank. In the main storage tank the product is kept just above the melting point to reduce the risk of degradation if the coating agent is heat-sensitive. From this main tank the product is pumped via filters in heated pipelines to a day storage tank for heating to the spraying temperature. Instead of a day-tank, heating can be done online before the dosage pump. The product is pumped from the day-tank via filters by a metering pump controlled by the production rate, to the nozzle. To ensure an even pressure a pump with multiple heads is recommended, or a hydraulic accumulator is fitted to the pipe.
Other important factors are the choice of and position of the nozzle/nozzles in the coating drum, the rolling bed effect produced in the coating drum and residence time.
It is important to place the spraying nozzle correctly in relation to the rolling bed of the fertiliser, which is created by the baffles inside the coating drum. Either hollow cone atomising nozzles or flat spray nozzles can be used but, with the latter, extra care must be taken when placing the nozzle inside the coating drum.
When no coating drum is available for spraying a liquid coating agent, then an alternative could be to spray at the end of the cooling drum. If possible, a rolling bed should be arranged at the end of the cooling drum to allow for proper mixing. One disadvantage of spraying into a cooling drum is that spray mist may be lost into the air, which could make it impossible to use this alternative.
The most widely used method, if no coating drum is at place, is to spray in a fall-shaft between two conveyor belts. Spraying is done with one or two nozzles on the falling curtain. To obtain as good mixing as possible, spraying should be done in the first fall-shaft after production allowing for passing as many points of mixing as possible before storage or bagging. This spraying place should be built-in to avoid spray mist from entering the surroundings and good ventilation is advised.
When space allows, a better alternative is to install a small box and then spray with two nozzles, one on each side of the falling curtain of fertiliser. By doing so, an even better distribution is obtained as well as reduced spray mist in the surroundings.
When dosing solid coating agents for melt application, the dosage system is much simpler. Most important is that the fertiliser at the dosage point is hot, at least 10-15°C above the melting point of the additive, and that cooling is available after dosage. The additive is fed from a hopper by a screw, controlled by the production rate, onto a conveyor belt, or directly into the coating drum. If no such drum is available before the cooler, it is possible to use the first part of the cooling drum. In this case the lifters of the cooler need to be changed in order to create a rolling bed to ensure a better distribution of the coating agent.
If neither a coating nor a cooling drum is available, dosage might be done directly on the conveyor belt. It is then important that mixing devices are placed in the top layer of the fertiliser on the conveyor belt to ensure some mixing. Also the application point should be placed before the fall-shafts in order to give further mixing, and hence improved distribution of the solid coating agent.
UV-light can be used to check the efficiency of spraying and the coating drum. Comparison of untreated and treated fertiliser samples will give an indication of the distribution of the coating agent.
Source from https://fertechinform.org/