
Exhibition time: 17-19 March, 2026 Shanghai, China
 中文
中文

Exhibition time: 17-19 March, 2026 Shanghai, China
 中文
中文
by Christian Schmalz, Marketing Director, UlexAndes-USA
Despite requiring small quantities, micronutrients play a complex role in crop development and health. Manganese (Mn) as a critical micronutrient is widely accepted, playing a key role in several biochemical reactions; particularly photosynthesis.

Manganese: The element
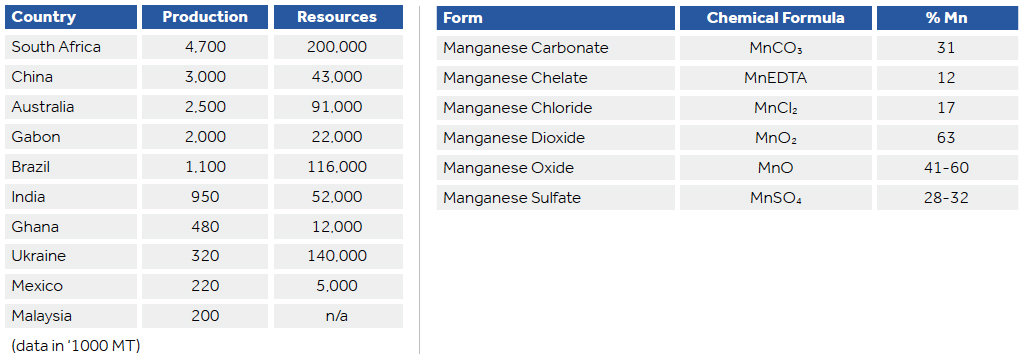
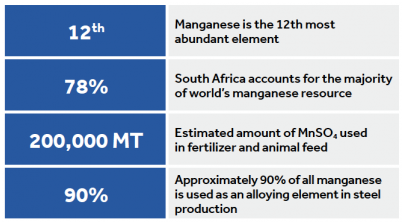
The use of manganese dates back to the Stone Age where it can be seen in cave paintings. Belonging to the transition series of elements, manganese is second only to iron (Fe) in abundance. It commonly forms several minerals including pyrolusite (MnO2), rhodochrosite (MnCO3), manganite (MnO). It is also widely distributed as an accessory element.
Manganese ore containing more than 20% manganese has not been produced domestically since 1970. South Africa accounts for over 75% of the worlds identified resources, with Ukraine accounting for another 10%.
Manganese is closely related to iron, and to a lesser extent zinc, copper, magnesium and calcium. Due to their similarity, manganese and iron can compete for plant uptake.
Coincidently, their deficiencies/toxicities also have similar visual symptoms: interveinal chlorosis of the young leaves. The only true way to distinguish between iron and manganese deficiency is through diagnostic testing [which is also a NOP/OMRI requirement for organic use].
The behavior of Mn in soil is very complex, and is impacted greatly by the pH conditions and organic matter. In addition, the level of Mn is controlled by the geochemistry of the source rock and the redox conditions of the environment.
Manganese: Agronomy
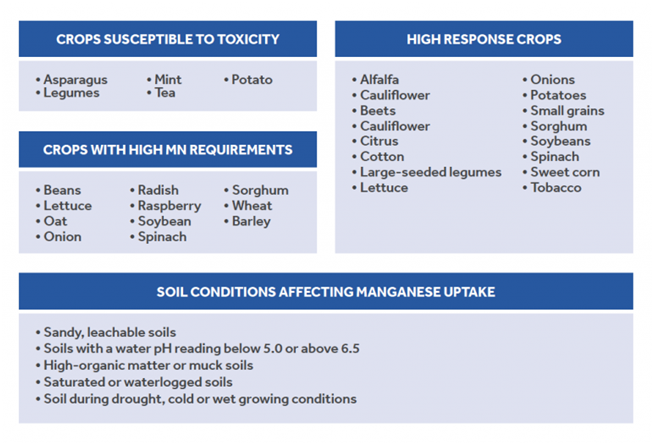
Although plants rely on manganese primarily for photosynthesis, a variety of other biological functions require manganese including chlorophyll synthesis, nitrate assimilation, lipid metabolism, enzyme activationand vitamin formation. In photosynthesis, Manganese (Mn) plays an important electron transport role in redox reactions. It also plays a role in building carbohydrates and metabolizing nitrogen, especially in corn. As a cofactor, Mn reportedly activates over 35 enzymes necessary for plant health and vigor including lignin, phytoalexins, peroxidase and superoxide dismutase. Impairment of these functions in crops increases susceptibility to pathogen attack and reduces stress tolerance. Most of the Manganese used by the plant comes from the soil solution and reaches plant roots by a process called mass flow and diffusion. Deficiencies occur in neutral-to-high pH soils
Manganese: Application
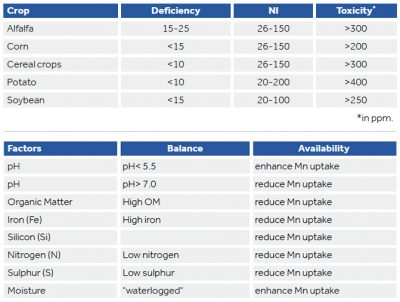
Manganese sulphate is the most common form used in plant and animal feed industries. Manganese fertilization is recommended if soil tests < 10ppm. In-row bands (1-5lb/A) or foliar (1-2 lb/A) applications in soil corrected to the proper pH are typically recommended. Broadcast applications are not recommended because manganese that is not concentrated quickly converts to unavailable formats. As manganese toxicity often results from an acidic soil pH, lime or dolomite can be applied to raise the pH. Correcting drainage issues that have lead to waterlogging will also remove one element impacting manganese toxicity.
Toxicity
Manganese (Mn) toxicity is associated with waterlogged soils where anaerobic conditions are present, or acidic soil conditions where the pH < 5.5. Anaerobic conditions cause higher oxides of manganese to be reduced to plant-available Mn2+. At a pH<5.5, manganese and oxygen react spontaneously. Similar to boron toxicity, manganese toxicity begins with the burning of the tips and margins of older leaves or as necrotic spots. Due to an induced iron deficiency, manganese toxicity also frequently causes pale or yellow patches between veins in younger plants (interveinal chlorosis). These necrotic lesions can spread until the entire leaf area is compromised. Eventually leaves turn yellow and are shed.
Deficiency
Manganese deficiency negatively impacts dry matter and yields, weakens resistance to pathogens and reduces drought and heat tolerance. Due to low phloem mobility in plants, manganese deficiency can mimic iron; first symptoms develop in younger leaves in the form of interveinal chlorosis. Deficiencies are most likely to occur in neutral-to-high pH soils that are also high in organic matter or as a result of low fertilizer application rate.
Manganese: Process and production
Most manganese ore mining occurs in open pits. Although it can be mined from the ocean floor, land-based mining dominates using compact mining equipment and advanced technology. Only ores containing greater than 35% manganese are considered commercially exploitable. Hydrometallurgical and electrolytic processes produce pure manganese. Manganese salt, primarily MnSO4, is the form used in the agriculture and feed industries. Complicated chemical redox reactions are necessary to produce manganese sulphate (MnSO4) from manganese dioxide (MnO2) and sulphur dioxide (SO2).
