
Exhibition time: 17-19 March, 2026 Shanghai, China
 中文
中文

Exhibition time: 17-19 March, 2026 Shanghai, China
 中文
中文
Plant nutrients are one of the environmental factors essential for crop growth and development. Nutrient management is crucial for optimal productivity in commercial crop production. Those nutrients in concentrations of ≤ 100 parts per million (ppm) in plant tissues are described as micronutrients and include iron (Fe), zinc (Zn), manganese (Mn), copper (Cu), boron (B), chlorine (Cl), molybdenum (Mo), and nickel (Ni). Micronutrients such as Fe, Mn, Zn, and Cu are easily oxidized or precipitated in soil, and their utilization is, therefore, not very efficient. Chelated fertilizers have been developed to increase micronutrient utilization efficiency.
What is chelated fertilizer?
The word chelate is derived from the Greek word chelé, which refers to a lobster's claw. Hence, chelate refers to the pincer-like manner in which a metal nutrient ion is encircled by the larger organic molecule (the claw), usually called a ligand or chelator. Each ligand, when combined with a micronutrient, can form a chelated fertilizer. Chelated micronutrients are protected from oxidation, precipitation, and immobilization in certain conditions because the organic molecule (the ligand) can combine and form a ring encircling the micronutrient. The pincer-like manner in which the micronutrient is bonded to the ligand changes the micronutrient's surface property and favors the uptake efficiency.
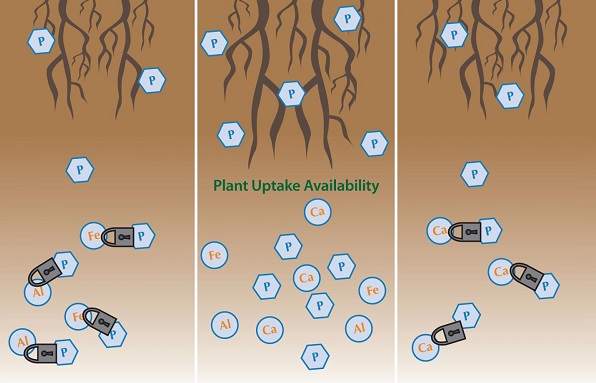
Why is chelated fertilizer needed?
Because soil is heterogeneous and complex, traditional micronutrients are readily oxidized. Chelation keeps a micronutrient from undesirable reactions in solution and soil. The chelated fertilizer improves the bioavailability of micronutrients such as Fe, Cu, Mn, and Zn, and in turn contributes to the productivity and profitability of commercial crop production. Chelated fertilizers have a greater potential to increase commercial yield than regular micronutrients if the crop is grown in low-micronutrient stress or soils with a pH greater than 6.5. In order to grow a good crop, crop nutrient requirements (CNRs), including micronutrients, have to be satisfied first from the soil. If the soil cannot meet the CNR, chelated sources need to be used. This approach benefits the plant without increasing the risk of eutrophication.
Several factors reduce the bioavailability of iron, including high soil pH, high bicarbonate content, plant species (grass species are usually more efficient than other species because they can excrete effective ligands), and abiotic stresses. Plants typically utilize iron as ferrous iron (Fe2+). Ferrous iron can be readily oxidized to the plant-unavailable ferric form (Fe3+) when soil pH is greater than 5.3 (Morgan and Lahav 2007). Iron deficiency often occurs if soil pH is greater than 7.4. Chelated iron can prevent this conversion from Fe2+ to Fe3+.
Applying nutrients such as Fe, Mn, Zn, and Cu directly to the soil is inefficient because in soil solution they are present as positively charged metal ions and will readily react with oxygen and/or negatively charged hydroxide ions (OH-). If they react with oxygen or hydroxide ions, they form new compounds that are not bioavailable to plants. Both oxygen and hydroxide ions are abundant in soil and soilless growth media. The ligand can protect the micronutrient from oxidization or precipitation.
From An Article By Guodong Liu,Edward Hanlon, And Yuncong Li,University Of Florida
