
Exhibition time: 17-19 March, 2026 Shanghai, China
 中文
中文

Exhibition time: 17-19 March, 2026 Shanghai, China
 中文
中文
Iron deficiency is a limiting factor of plant growth. Iron is present at high quantities in soils, but its availability to plants is usually very low, and therefore iron deficiency is a common problem.
IRON AVAILABILITY TO PLANTS
Although most of the iron on the earth crust is in the form of Fe3+, the Fe2+ form is physiologically more significant for plants. This form is relatively soluble, but is readily oxidized to Fe3+, which then precipitates.
Fe3+ is insoluble in neutral and high pH, making iron unavailable to plants in alkaline and in calcareous soils. Furthermore, in these types of soil, iron readily combines with phosphates, carbonates, calcium, magnesium and hydroxide ions. In such types of soils, it is recommended to use iron chelates
IRON UPTAKE BY PLANTS
Plants uptake iron in its oxidized forms, Fe2+ (ferrous form) or Fe3+ (ferric form).
Plants use various iron uptake mechanisms. One of these is the chelation mechanism – the plant releases compounds called siderophores which bind iron and enhance its solubility. This mechanism also involves bacteria.
Another mechanism involves the release of protons (H+) and reductants by the plant roots, to lower pH levels in root zone. The result is increased iron solubility.
In this respect, choice of the form of nitrogen fertilizer is significant. Ammonium nitrogen increases proton release by roots, thus lowering pH and facilitating iron uptake.
Nitrate nitrogen enhances the release of hydroxide ions that increase pH in the root zone and counteract efficient iron uptake.
New roots and root hairs are more active in iron uptake, therefore it is imperative to maintain a healthy active root system. Any factor interfering with root development interferes with iron uptake.
MANAGING IRON DEFICIENCIES
When iron deficiency is identified, it can be treated in the short term by applying a foliar spray of iron, but the best course of action is prevention. Therefore, the grower should identify the real cause of the deficiency and treat it, in order to prevent the problem from occurring in the future.
Often, iron deficiency does not indicate insufficient iron supply. It may also be related to various conditions that may affect iron availability. For example: carbonate levels in the soil, salinity, soil moisture, low temperature, concentration of other elements (e.g. competitive microelements, phosphorus, calcium) etc.
Evaluating these factors and correcting them can save a great deal of money spent on ineffective and unnecessary iron applications.
IRON FERTILIZERS
Iron can be applied as ferrous sulfate or in a chelated form.
Ferrous sulfate (FeSO4) contains about 20% iron. This fertilizer is inexpensive and is mainly used for foliar spraying. Applied to soil, it is often ineffective, especially in pH above 7.0, because its iron quickly transforms to Fe3+ and precipitates as one of the iron oxides.
Iron chelates. Chelates are compounds that stabilize metal ions (in this case – iron) and protect them from oxidation and precipitation.
Iron chelates consist of three components:
Fe3+ ions
A complex, such as EDTA, DTPA, EDDHA, amino acids, humic-fulvic acids, citrate.
Sodium (Na+) or ammonium (NH4+) ions
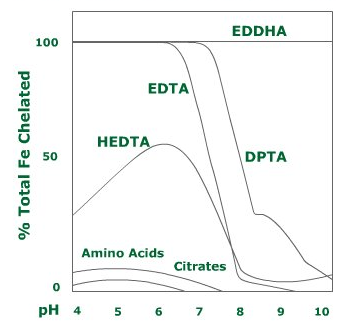
Different chelates hold iron ions in different strengths at different pH levels. They also defer in their susceptibility to iron replacement by competitive ions. For example, at high concentrations, calcium or magnesium ions may replace the chelated metal ion.
Fe-EDTA – This iron chelate is stable at pH below 6.0. Above pH of 6.5, nearly 50% of the iron is unavailable. Therefore this chelate is ineffective in alkaline soils. This chelate also has high affinity to calcium, so it is advised not to use it in calcium-rich soils or water.
Note that EDTA is a very stable chelate of micro-elements, other than iron, even in high pH levels.
Fe-DTPA – this iron chelate is stable in pH levels of up to 7.0, and is not as susceptible to iron replacement by calcium.
Fe-EDDHA – this chelate is stable at pH levels as high as 11.0, but it is also the most expensive iron chelate available.
In soilless media and hydroponics, pH monitoring of water and media is relatively easier than in soils. When regular testing is performed, and pH control is adequate, it is possible to prefer the inexpensive, less stable iron chelates.
On the other hand, in alkaline soils, where it is difficult to effectively decrease pH levels, it is advised to use more stable iron chelates, such as EDDHA.
From SMART FERTILIZER SOFTWARE
