
Exhibition time: 17-19 March, 2026 Shanghai, China
 中文
中文

Exhibition time: 17-19 March, 2026 Shanghai, China
 中文
中文
The pH of the soil and the feeding water are essential aspects of a good feeding plan. pH does not have a direct effect on the plant, but it does directly affect the availability of the nutrients for the plant. The plant, in turn, can also influence the pH of the soil in the area close to the roots, as we will discuss later in this article.
To better understand the effect of pH on your crop yields, we first need to define pH. The pH scale, the standard measurement of acidity, was developed by the head of Carlsberg Laboratory’s Chemical Department in 1909. It basically means ‘the power of hydrogen’, because the scale provides a simple and universal measurement of the amount of hydrogen ions there are in a solution. These ions affect its acidity and how the solution will react chemically. pH is defined as the negative logarithm of the hydrogen ion concentration. It is a result of the presence of anions (negatively charged nutrients) and cations (positively charged nutrients).
The pH scale goes from 0 (acid) to 14 (alkaline) with pH 7 as the neutral point.
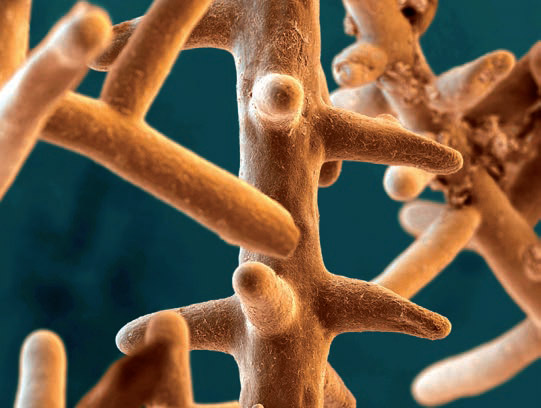
Figure 1: This is a colored scanning electron micrograph (SEM) of mycorrhizae; a symbiotic association between a soil fungus and the roots of a vascular plant. The fungus is able to access nutrient forms unavailable to the plant, process them and pass them on to the roots. Mycorrhizae prefer a slightly acidic environment for optimum growth.
The rhizosphere is the narrow region of soil that is directly influenced by root secretions and associated soil microorganisms. Plants respond to nutrient deficiency by altering their root morphology, recruiting the help of microorganisms and changing the chemical environment of the rhizosphere. Components in root exudates help plants to access nutrients by acidifying or changing the redox conditions within the rhizosphere or chelating directly with the nutrient. Exudates can liberate nutrients via the dissolution of insoluble mineral phases or desorption from clay minerals or organic matter, whereby they are released into the soil in solution and can then be taken up by the plant.
When preparing a nutrient solution, a grower ensures that the pH of the water is within a certain range. This range will preferably be that at which most nutrients are available to the plant, which is 5.2 - 6.2. If necessary the pH of the fertilizer solution can simply be adjusted by adding an acid to lower the pH or a base to increase it (read more about it in pH acidity: what it does to your plants). But in the rhizosphere, the direct surrounding of the living roots, things become very different. The roots excrete many substances that alter the pH in the substrate.

Figure 2: Each soil particle contains a net negative
electrical charge and therefore has the ability to
attract and hold positively charged elements like
potassium and calcium. These elements are attracted
and held to the surface of the soil particles like a magnet.
Clay and organic matter have a higher net negative electrical
charge and therefore have more capacity to hold positively
charged ions or cations. Negatively charged ions such
as nitrate and phosphate will normally be repelled.
The pH in the rhizosphere can be very different from the pH which is measured in the nutrient solution. The principal cause of this is that the plant has to remain ‘neutral’. When they are dissolved in water, all nutrients are present as ions. Those ions always have a positive or a negative charge. Positively charged ions, like K+, are called cations. Negatively charged ions, like NO3 -, are called anions. Some nutrients can be present in multiple forms. For example phosphates, which can occur as PO4 3- , HPO4 2- and H2PO4 -. However, only this last form can be taken up by the roots. The surface of the root is negatively charged. In this state, the negatively charged ions such as H2PO4 - will be repelled from the root surface like two magnets that have the same pole.
Plants have developed several ways of facilitating anion uptake. For every anion the plant takes up, it excretes an anion such as a hydroxide (OH-) or bicarbonate ion (HCO3 -). Similarly, for every cation it takes up, the plant excretes a cation as a H+. In this way, the plant’s charge remains balanced. However, a side-effect of this is that the excreted ions influence the pH of the rhizosphere in the substrate. By excreting a cation, the pH near the roots decreases (it becomes more acid). The excretion of anions will raise the pH near the roots (it becomes more alkaline).
It is well-known that nitrogen fertilizers have an effect on the pH near the roots. That insight is important because the plant takes up so much nitrogen that the effect can be considerable. But this effect occurs with every nutrient or fertilizer.
As a grower, you can add nitrogen in different forms. Ammonium (NH4 +) has an acidic effect in the soil. Nitrate (NO3 -) has an alkaline effect. One might easily assume that the answer to this is to fertilize with ammonium nitrate (NH4NO3). But it isn’t that simple. The ammonium will be taken up much faster by the plant compared to nitrate, and the result in the end will be acidification of the soil. All these reactions need to be taken into account because every nutrient has its own optimum pH-range in the soil with respect to plant availability. For some elements, this is a narrow pH-range and simply measuring the pH in the nutrient solution will not tell you what is really happening down in the rhizosphere.
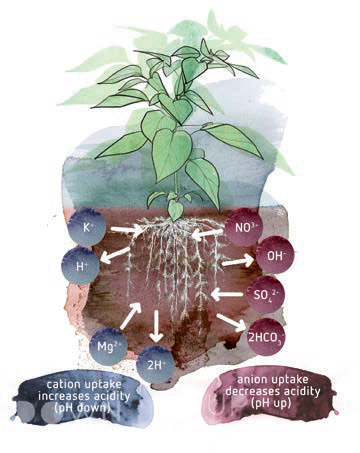
Figure 3: This image shows you that for every cation (blue)
that a plant takes up, it excretes a cation as H+. For
every anion (red) a plant takes up, it releases a
hydroxide (e.g. OH-) ion. In this way, the plant’s net charge
always remains in balance. A side-effect of this is that
the excreted ions influence the pH of the rhizosphere
in the substrate. When the plant excretes a cation,
the pH near the roots decreases. The excretion
of anions will raise the pH near the roots.
In the past, it became clear that roots excrete many substances in order to influence the soil-life directly around the surface of the roots. These substances are known as ‘exudates’. The main exudates are sugars and organic acids. Acids such as citric acid, oxalic acid and malic acid are present to a large extent in the cell moisture of the roots. These elements also can have an influence on the pH in the soil but how strong this influence is will vary in every plant.
If the acids are excreted from the roots, they are dissolved as anions and will make the soil near the root more alkaline, like other anions. Usually these exudates will have a minor influence on the pH compared to the strong effect of H+ -ion excretion. What is remarkable, however, is that not every piece of the root system acts in the same way. At the tip of the root, more H+ -ions are excreted, while a bit further down the root, more anions are excreted. This is probably connected to the differences in the uptake of fertilizers.
