
Exhibition time: 17-19 March, 2026 Shanghai, China
 中文
中文

Exhibition time: 17-19 March, 2026 Shanghai, China
 中文
中文
 Soil contains high amount of silicon, ranging from 50 to 400 grams per kilogram, making it the most abundant element among solid metals in soil. The demand and importance of silicon fertilizer have led to numerous discussions, highlighting it as the fourth most essential element for crops and its well-known multiple benefits. Due to the wide range of benefits brought about by silicon fertilizer, including stress resistance, yield increase, soil improvement, and quality enhancement, the marketing of related silicon fertilizer products has created a lot of ambiguous space. This article aims to provide readers with an understanding to the mechanism of silicon, enabling better assessment and identification on the efficacy of products.
Soil contains high amount of silicon, ranging from 50 to 400 grams per kilogram, making it the most abundant element among solid metals in soil. The demand and importance of silicon fertilizer have led to numerous discussions, highlighting it as the fourth most essential element for crops and its well-known multiple benefits. Due to the wide range of benefits brought about by silicon fertilizer, including stress resistance, yield increase, soil improvement, and quality enhancement, the marketing of related silicon fertilizer products has created a lot of ambiguous space. This article aims to provide readers with an understanding to the mechanism of silicon, enabling better assessment and identification on the efficacy of products.
First of all, what form of silicon is utilized by crops? The price of silicon fertilizer depends on what aspects? Silicates is the typical form which is utilized by crops. Silicates dissociate into silicate ions in water, further forming silicic acid (H2SiO3) or silicate di-ions (H2SiO4-), depending on the pH value of the solution. Both forms are available for plant uptake. Silicon fertilizers are mainly derived from minerals, including silicate minerals such as zeolite, quartz (silicon dioxide SiO2), feldspar, and pyroxene. Another source is diatomaceous earth, commonly known as diatomite, which is primarily composed of diatoms, their remains, and other organic and inorganic substances. Industrial by-products such as calcium silicate (gypsum) and sodium silicate (sodium carbonate residue) can also be used. Therefore, understanding the source of silicon fertilizers can provide insights into their product characteristics and impurity content, which can help evaluate their effectiveness. Among the various sources, zeolite is a common silico-aluminophosphate mineral formed by the combination of silicon-oxygen tetrahedra and aluminum-oxygen octahedra. Its chemical formula is usually (Na2,K2,Ca)Al2Si8O20•6H2O. The silicate content of zeolite depends on the specific type of zeolite mineral, such as clinoptilolite, phillipsite, greensand, or chabazite, and can range from 40% to 70%. Therefore, price differences also arise due to variations in their specific applications. Understanding the source and type of silicon fertilizers allows us to comprehend the differences in silicon content in terms of quality and quantity.
Now, let us walk through the mechanism of action of silicon fertilizer from several aspects.
Soil Amendment Function
The mechanism of silicon fertilizer in soil amendment involves the interaction and adsorption processes between silicon ions (Si4+) and the surface of soil particles. When silicon ions in the fertilizer come into contact with soil particles, ion exchange and adsorption occur. Silicon ions can form adsorption complexes with hydroxides of aluminum, iron oxides, and clay minerals on the soil particle surfaces. This adsorption process is achieved through mechanisms such as electrostatic attraction, surface coordination, and chemical reactions. In the context of fertilizers, this mechanism improves soil cation exchange capacity and enhances nutrient retention in soil.
Improvement of Leaf Physical Properties
Upon entering the plant, monomeric silicic acid readily loses a proton (hydrogen ion) to form an anion. These anions then bond together to form dimers, oligomers, and eventually polymeric silicic acid particles. This structure formed inside the plant is known as "silicification." The process of silicon accumulation in algae is relatively well-studied. In the early stages, silicon appears in a gel-like form within the cells. As the algae mature, circular granules of silicon start to form on the surfaces and backs, with the highest content reaching up to 0.5% of the total matrix. Adding silicon fertilizer increases the accumulation of cuticle layers and dry matter in leaves by up to 85% and 46%, respectively. However, this process requires sufficient calcium and pectin in cell. The silicon content in leaf trichomes is significantly higher, up to 75% higher compared to the control group. The addition of silicon also reduces leaf deformation rates and leaf damage by 15% and 25%, respectively. It also significantly lowers the infection rates of powdery mildew and gray mold.
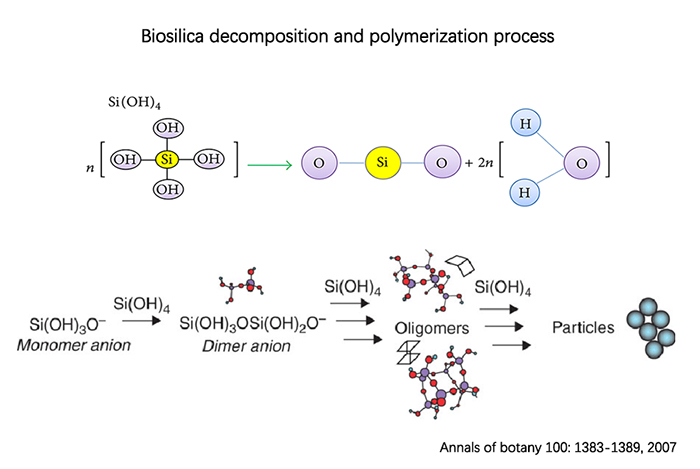
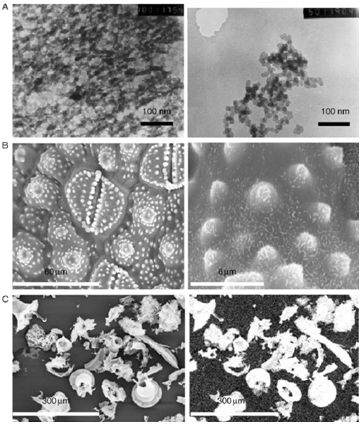
Accumulation of silica in algae cell
(Source: Annals of Botany 100: 1383–1389, 2007)
Mechanism in Alleviating Salt Stress
The mechanism of silicon fertilizer in the alleviation of salt stress in crops involves an understanding of cellular structures. Silicon can reduce cellular dehydration and shrinkage caused by high osmotic stress from salt ions. The main mechanism is the increase in the potassium/sodium ratio on the cell membrane. In high-salinity environments, the efflux of potassium ions and the influx of sodium ions stabilize the membrane potential, increase membrane enzyme activity, and reduce cytoplasmic loss. Under severe salt stress, silicon can dissolve soluble proteins on the leaf surface to compensate for the loss of osmotic pressure in the cell membrane and prevent cell death. Various redox enzymes are involved in this process.
Heavy Metal Resistance
Research on the effects of silicon on heavy metals in plants first appeared in barley crops in 1957. Silicon does not affect the total amount of manganese absorbed by barley, but it allows manganese to be evenly distributed on the leaf surface, reducing localized leaf toxicity. Substances in plant cells circulate through symplastic pathways and apoplastic pathways. Silica bodies can form physical barriers in the apoplastic space, reducing the rate of cadmium transport and the accumulation and distribution of cadmium in the cytoplasm. Additionally, silicon has a co-precipitation mechanism with cadmium in cell walls. In studies on maize, heavy metals such as cadmium and zinc co-precipitate with silicon in various cells such as the root epidermis, outer layer, inner layer, and xylem, leading to detoxification. Japanese researchers have also found that silicon can precipitate heavy metals in their oxidized state.
For heavy metals outside the cell, silicon directly forms covalent bonds with them, rendering their inactivation. For heavy metals that have entered the cell, silicon can increase the activity of triphosphatase ATPase and pyrophosphatase Ppase. These two enzymes are involved in energy release and can transport heavy metals from organelles to vacuoles, thereby preventing further damage to other organelles. In summary, the mechanism can be summarized as "intracellular sequestration and extracellular inactivation" of heavy metals.
The formation mechanism and physiological mechanisms of silicon in plants involve various physiological, biochemical, and molecular processes that have been well understood. The absorption of silicon requires specific transport proteins and is supported by energy mechanisms, thus, low temperatures and metabolic inhibitors can inhibit silicon transport. Amino acids such as serine serve as mediators for the formation of complex silicates, including polysilicates, from monosilicic acid. However, silicon is not a universal solution. Monosilicic acid is chemically inactive and can form colloidal particles of polysilicic acid. Excessive polysilicic acid can interfere with soil structure formation and water-holding capacity. Due to limitations in atomic electron structure, silicon cannot form chemical bonds with many other types of atoms, which restricts its direct involvement in many important biochemical metabolic reactions. Nevertheless, based on the current understanding of silicon's functions, it is sufficient to bring about a broad spectrum of comprehensive benefits.
Regarding the multi benefits of silicon fertilizer, there are some new applications based on the properties of silicon and innovative formulations. Nanosilicon has entered the market recently, addressing the challenges from common silicon fertilizer. Conventional silicon fertilizers, commonly referred to as micron-scale (µm) silicon, have been surpassed by nanosilicon with particle sizes ranging from 1 to 100 nanometers (nm), offering greater potential for widespread applications in agriculture. Firstly, nanosilicon particles can serve as adjuvants for insecticides, adhering to pests and accelerating the penetration of pesticides. Nanosilicon also serves as a novel delivery system for fertilizers. With its carrier function, nanosilicon particles have a larger surface area per unit mass. Consequently, they provide a greater adsorption surface area, protecting fertilizers from environmental losses and effectively delivering them to the root zone of plants. Additionally, nanosilicon acts as a plant protectant by forming a physical barrier, enhancing plant resistance against pathogens and stress factors.
The conventional liquid silicon fertilizers used in field crops also encountered certain challenges and drawbacks. One issue is the occurrence of precipitation or sedimentation in liquid silicon fertilizers. Additionally, liquid silicate solutions often have an alkaline pH, requiring pH adjustment to fit different soil conditions and crop requirements. Nanoscale liquid silicon addresses these concerns. With its nearly neutral characteristics, nanoscale silicon resolves the compatibility issues with most pesticides. Furthermore, nanoscale silicon, falls into the category of emulsions, displaying a property of suspension and thereby addressing the instability during formulation. One innovative aspect of formulation is the combination of silicon (silicon-aluminum silicate) with nutrient elements or microbial agents, which elevates the application of silicon fertilizers. The application technology with excellent carrier functionality serves as a crucial foundation for reducing fertilizer usage and enhancing efficiency, drawing inspiration mainly from the practices in animal husbandry. By emphasizing its ability to adsorb harmful substances, the application foundation shifts towards fertilization preservation and reduction.

Co-cultivation of rice and crayfish
In China, several imported fertilizer products related to this concept have demonstrated their efficacy greatly in fertilizer usage reduction, while also increasing efficiency and improving output quality over the past 6-7 years. The combination of silicon fertilizer and microbial agents is an ideal synergetic effect between inorganic and organic components. Common silicon fertilizer varieties, when combined with Bacillus species, can protect microbial agents from inactivation and prolong their release effectiveness in the soil. In practical applications, research data shows that nanoscale silicon can serve as a foliar barrier for rice plants against cadmium absorption. Experimental results indicate that cadmium content in the treated groups decreased to within national standards, with a maximum reduction of 64.47%. Moreover, rice yield increased by 9.88%, achieving dual benefits of increased productivity and food safety. Unpublished data from the production of pollution-free Sichuan pepper also indicates that, compared to conventional fertilizers, the use of granular silicon fertilizer reduced mercury, lead, and cadmium levels by 83%, 69%, and 38%, respectively. In other domestic applications, the co-cultivation of rice and crayfish using silica-based compound fertilizers provides not only nutrition for rice but also meets the water purification needs of rice-crayfish fields, achieving a good yield for rice and crayfish.
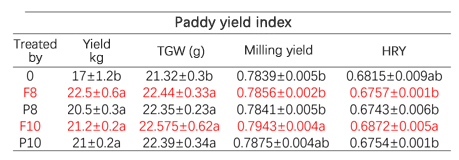
Source: AgroNews