
Exhibition time: 17-19 March, 2026 Shanghai, China
 中文
中文

Exhibition time: 17-19 March, 2026 Shanghai, China
 中文
中文
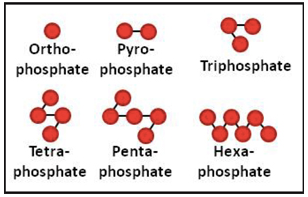
Key words of the passage: polyphosphate; production; fertilizer
Despite phosphorus (P) availability from geologic deposits distributed around the globe, widespread P deficiency in soils limits the growth and productivity of plants in many parts of the world. Because of this, growers commonly add this nutrient to their fields to improve crop yield and quality. Polyphosphate is an excellent liquid P fertilizer used to increase agricultural production.
Phosphoric acid is the starting material for most commercial phosphate fertilizers. However, its acidity and some of its chemical properties make this material difficult to apply directly.
The most common ammonium polyphosphate fertilizers have a N-P₂O₅-K₂O (nitrogen, phosphorus and potassium) composition of 10-34-0 or 11-37-0. Polyphosphate fertilizers offer the advantage of a high nutrient content in a clear, crystal-free fluid that remains stable within a wide temperature range and stores well for long periods. A variety of other nutrients mix well with polyphosphate fertilizers, making them excellent carriers of micronutrients typically needed by plants.
A single phosphate molecule is called an orthophosphate. When phosphoric acid and ammonia are reacted, water is driven off and orthophosphates begin to link together, chainlike, to form a “polyphosphate” fluid fertilizer. Each linkage of phosphate molecules has its own name deriving from its length, although polyphosphate is the general term that includes all of these linked molecules.
Source: https://www.cropnutrition.com/resource-library/polyphosphate
