
Exhibition time: 17-19 March, 2026 Shanghai, China
 中文
中文

Exhibition time: 17-19 March, 2026 Shanghai, China
 中文
中文
Nitrate nitrogen (NO₃⁻-N) and ammonium nitrogen (NH₄⁺-N) are two primary forms of nitrogen used in agriculture, each with distinct characteristics and applications:
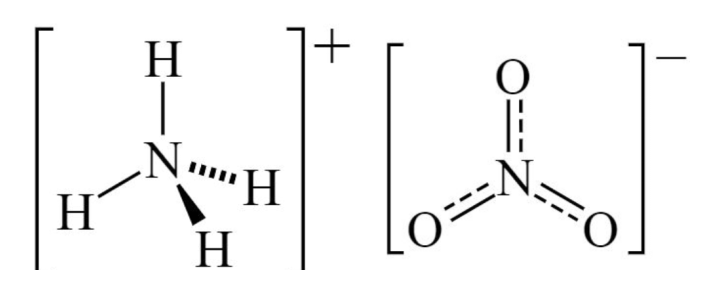
1. Absorption and Mobility:
· Nitrate Nitrogen: Highly mobile in soil, easily absorbed by plant roots, and suitable for most crops, especially during rapid growth phases. It moves freely with soil water, making it ideal for fertigation and top-dressing.
· Ammonium Nitrogen: Less mobile, as it binds to soil particles, reducing leaching losses. It is absorbed more slowly and is preferred for crops in early growth stages or in soils with low cation exchange capacity.
2. Soil and Environmental Conditions:
· Nitrate Nitrogen: Performs better in well-aerated, neutral, or alkaline soils. However, it is prone to leaching in sandy or high-rainfall areas, potentially causing environmental issues like groundwater contamination.
· Ammonium Nitrogen: Preferred in acidic soils or waterlogged conditions (e.g., rice paddies), as it is less susceptible to leaching. It can be converted to nitrate via nitrification, depending on soil conditions.
3. Application Timing and Method:
· Nitrate Nitrogen: Often used in fast-acting fertilizers (e.g., calcium nitrate, potassium nitrate) for immediate plant uptake, suitable for quick nitrogen boosts during vegetative growth.
· Ammonium Nitrogen: Found in fertilizers like ammonium sulfate or urea, providing a more sustained release. It’s often applied before planting or in split applications to ensure steady nitrogen supply.
4. Crop Suitability:
· Nitrate Nitrogen: Favored by crops like vegetables, fruits, and cereals that require rapid nitrogen availability.
· Ammonium Nitrogen: Preferred for crops like rice or in situations where prolonged nitrogen availability is needed, such as in cooler climates where nitrification is slower.
5. Risks and Considerations:
· Nitrate Nitrogen: Overuse can lead to leaching and environmental pollution. It requires careful management in high-precipitation areas.
· Ammonium Nitrogen: Excessive application may cause soil acidification or ammonia volatilization, especially in high-pH or dry soils.
In summary, nitrate nitrogen is used for quick uptake and mobility, while ammonium nitrogen is chosen for stability and sustained release, with the choice depending on soil type, crop needs, and environmental factors.